
Hepatic Tumor Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 75+ Companies Working in the Domain
Hepatic tumors are abnormal growth of liver cells on or in the liver. The Hepatic Tumor market is driven by several key factors reflecting the increasing incidence of liver cancer, advancements in treatment options, and the growing emphasis on personalized medicine and combination therapies. One of the primary drivers is the rising prevalence of risk factors associated with liver cancer, including chronic viral hepatitis (such as hepatitis B and C), non-alcoholic fatty liver disease (NAFLD), alcohol abuse, and metabolic disorders.
/EIN News/ -- New York, USA, July 30, 2024 (GLOBE NEWSWIRE) -- Hepatic Tumor Clinical Trial Pipeline Experiences Momentum: DelveInsight Estimates a Diverse Pipeline Comprising 75+ Companies Working in the Domain
Hepatic tumors are abnormal growth of liver cells on or in the liver. The Hepatic Tumor market is driven by several key factors reflecting the increasing incidence of liver cancer, advancements in treatment options, and the growing emphasis on personalized medicine and combination therapies. One of the primary drivers is the rising prevalence of risk factors associated with liver cancer, including chronic viral hepatitis (such as hepatitis B and C), non-alcoholic fatty liver disease (NAFLD), alcohol abuse, and metabolic disorders.
DelveInsight’s 'Hepatic Tumor Pipeline Insight 2024' report provides comprehensive global coverage of pipeline hepatic tumor therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the hepatic tumor pipeline domain.
Key Takeaways from the Hepatic Tumor Pipeline Report
- DelveInsight’s hepatic tumor pipeline report depicts a robust space with 75+ active players working to develop 75+ pipeline therapies for hepatic tumor treatment.
- Key hepatic tumor companies such as Can Fite Biopharma, MiNA Therapeutics, Medivir AB, Etnova Therapeutics Corp., SillaJen Biotherapeutics, Akeso Biopharma, Shanghai Junshi Biosciences, Genmab, Janssen Biotech, Bio-Thera Solutions, Coherus Biosciences, Inc., Genoscience, Onyx Pharmaceuticals, Qurient Co., Ltd., Kowa Company, Ltd., Eureka Therapeutics Inc., TRISALUS LIFE SCIENCES, Captor Therapeutics, Hepion Pharmaceuticals, Xbrane, Sinocelltech, Suzhou Zelgen Biopharmaceuticals, Likang Life Sciences, Myeloid Therapeutics, and others are evaluating new hepatic tumor drugs to improve the treatment landscape.
- Promising hepatic tumor pipeline therapies such as Namodenoson, MTL CEBPA, Fostroxacitabine bralpamide, ETN101, Pexastimogene devacirepvec, Cadonilimab, Toripalimab, Amivantamab, Bevacizumab biosimilar, SRF388, GNS561, PD-0332991, Q702, NIK-333, ET14020, Nelitolimod, CT-01, Rencofilstat, Nivolumab biosimilar, Finotonlimab, Donafenib, LK 101, MT 303, and others are under different phases of hepatic tumor clinical trials.
- In June 2024, Carisma Therapeutics Inc. announced the nomination of the first development candidate under its collaboration with Moderna, Inc. The development candidate is an in vivo CAR-M targeting Glypican-3 and is designed to treat solid tumors, including hepatocellular carcinoma. This nomination triggers a $2 million milestone payment to Carisma.
- In May 2024, Ascelia Pharma AB announced that its liver imaging drug candidate, Orviglance, significantly improved the visualization of focal liver lesions, successfully meeting the primary endpoint in the pivotal Phase III study SPARKLE.
- In February 2024, Immune-Onc Therapeutics, Inc. announced a Phase Ib/II clinical trial collaboration with Roche to evaluate Immune-Onc’s IO-108, a first-in-class antibody targeting LILRB2 (also known as ILT4), in combination with Roche’s atezolizumab and bevacizumab for the first-line treatment of patients with locally advanced or metastatic and/or unresectable hepatocellular carcinoma (HCC).
- In February 2024, Biosyngen announced that BST02 has been granted Fast Track Designation by the U.S. Food and Drug Administration (FDA) for the treatment of all types of liver cancer, including hepatocellular carcinoma and cholangiocarcinoma.
- In January 2024, CARsgen Therapeutics Holdings Limited announced that CT011 had achieved Investigational New Drug (IND) clearance from the National Medical Products Administration (NMPA) for patients with GPC3-positive stage IIIa hepatocellular carcinoma who are at high risk of recurrence after surgical resection.
- In December 2023, Bristol Myers Squibb and RayzeBio, Inc. announced a definitive merger agreement under which Bristol Myers Squibb will acquire RayzeBio for $62.50 per share in cash, for a total equity value of approximately $4.1 billion, or $3.6 billion net of estimated cash acquired.
- In November 2023, Target RWE announced today that the CQC and TARGET-Liver Disease (LD) partnership with the American Association for the Study of Liver Diseases (AASLD) has enrolled approximately 75,000 patients. The strategic partnership between Target RWE and AASLD is designed to fulfill the unmet need for a large, real-world registry of patients with chronic liver diseases.
Request a sample and discover the recent advances in hepatic tumor treatment drugs @ Hepatic Tumor Pipeline Report
The hepatic tumor pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage hepatic tumor drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the hepatic tumor clinical trial landscape.
Hepatic Tumor Overview
Liver tumors, also called hepatic tumors, represent a variety of growths that can develop in the liver. These growths may be benign or malignant and can originate from hepatocytes, the main liver cells, or other cell types within the liver. Common types of liver tumors include hepatocellular carcinoma (HCC), which arises from hepatocytes, and cholangiocarcinoma, which starts in the bile ducts within the liver. Other less frequent types are hepatic adenomas, focal nodular hyperplasia, and hemangiomas.
Hepatic tumors can result from various factors, including chronic liver diseases like hepatitis B or C, alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), and certain genetic disorders. Environmental factors, such as exposure to aflatoxins—toxins produced by molds found in grains and nuts—can also raise the risk. Lifestyle factors like obesity, smoking, and excessive alcohol consumption are linked to a higher risk of liver cancer.
Symptoms of liver tumors vary based on the tumor type and size but may include abdominal pain or discomfort, unexplained weight loss, loss of appetite, nausea, jaundice (yellowing of the skin and eyes), and an enlarged liver. Diagnosis typically involves imaging studies like ultrasound, CT scans, or MRI scans, and blood tests to evaluate liver function and detect tumor markers. Treatment options depend on the tumor type and stage and may include surgery, chemotherapy, radiation therapy, targeted therapy, and liver transplantation. Early detection and treatment are crucial for improving the prognosis and outcomes of patients with liver tumors.
Find out more about hepatic tumor treatment drugs @ Drugs for Hepatic Tumor Treatment
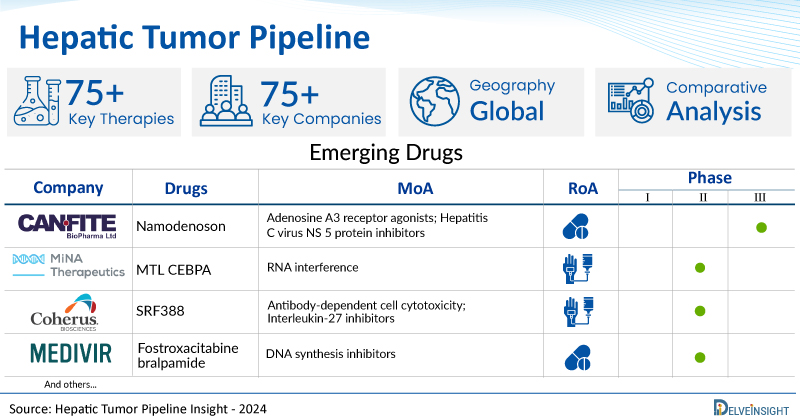
A snapshot of the Hepatic Tumor Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Namodenoson | Can Fite Biopharma | Phase III | Adenosine A3 receptor agonists; Hepatitis C virus NS 5 protein inhibitors | Oral |
| MTL CEBPA | MiNA Therapeutics | Phase II | RNA interference | Intravenous |
| SRF388 | Coherus Biosciences, Inc. | Phase II | Antibody-dependent cell cytotoxicity; Interleukin-27 inhibitors | Intravenous |
| Fostroxacitabine bralpamide | Medivir AB | Phase I/II | DNA synthesis inhibitors | Oral |
| ETN101 | Etnova Therapeutics Corp. | Phase I | Fms-like tyrosine kinase 3 inhibitors; Platelet-derived growth factor beta receptor antagonists; Protein kinase inhibitors; Vascular endothelial growth factor receptor-2 antagonists | Oral |
Learn more about the emerging hepatic tumor pipeline therapies @ Hepatic Tumor Clinical Trials
Hepatic Tumor Therapeutics Assessment
The hepatic tumor pipeline report proffers an integral view of the hepatic tumor emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Hepatic Tumor Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Adenosine A3 receptor agonists, Hepatitis C virus NS 5 protein inhibitors, RNA interference, Antibody-dependent cell cytotoxicity, DNA synthesis inhibitors, Fms-like tyrosine kinase 3 inhibitors, Platelet-derived growth factor beta receptor antagonists, Protein kinase inhibitors, Vascular endothelial growth factor receptor-2 antagonists
- Key Hepatic Tumor Companies: Can Fite Biopharma, MiNA Therapeutics, Medivir AB, Etnova Therapeutics Corp., SillaJen Biotherapeutics, Akeso Biopharma, Shanghai Junshi Biosciences, Genmab, Janssen Biotech, Bio-Thera Solutions, Coherus Biosciences, Inc., Genoscience, Onyx Pharmaceuticals, Qurient Co., Ltd., Kowa Company, Ltd., Eureka Therapeutics Inc., TRISALUS LIFE SCIENCES, Captor Therapeutics, Hepion Pharmaceuticals, Xbrane, Sinocelltech, Suzhou Zelgen Biopharmaceuticals, Likang Life Sciences, Myeloid Therapeutics
- Key Hepatic Tumor Pipeline Therapies: Namodenoson, MTL CEBPA, Fostroxacitabine bralpamide, ETN101, Pexastimogene devacirepvec, Cadonilimab, Toripalimab, Amivantamab, Bevacizumab biosimilar, SRF388, GNS561, PD-0332991, Q702, NIK-333, ET14020, Nelitolimod, CT-01, Rencofilstat, Nivolumab biosimilar, Finotonlimab, Donafenib,LK 101,MT 303.
Dive deep into rich insights for new drugs for hepatic tumor treatment, visit @ Hepatic Tumor Drugs
Table of Contents
| 1. | Hepatic Tumor Pipeline Report Introduction |
| 2. | Hepatic Tumor Pipeline Report Executive Summary |
| 3. | Hepatic Tumor Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Hepatic Tumor Clinical Trial Therapeutics |
| 6. | Hepatic Tumor Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Hepatic Tumor Pipeline: Late-Stage Products (Phase III) |
| 8. | Hepatic Tumor Pipeline: Mid-Stage Products (Phase II) |
| 9. | Hepatic Tumor Pipeline: Early-Stage Products (Phase I) |
| 10. | Hepatic Tumor Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Hepatic Tumor Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Hepatic Tumor Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the hepatic tumor pipeline therapeutics, reach out @ Hepatic Tumor Treatment Drugs
Related Reports
Liver Cancer Epidemiology Forecast
Liver Cancer Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted liver cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Liver Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key liver cancer companies, including CStone Pharmaceuticals, SOTIO, Akeso Biopharma, GlaxoSmithKline, Pfizer, Eureka Therapeutics Inc., TriSalus Life Sciences, Inc., Tvardi Therapeutics, Incorporated, Provectus Pharmaceuticals, Qurient Co., Ltd., Merck Sharp & Dohme LLC, Boehringer Ingelheim, DNAtrix, Inc., Omega Therapeutics, Celldex Therapeutics, Lantheus Medical Imaging, Eisai Inc., Codiak BioSciences, Genentech, Inc., Coherus Biosciences, Inc., Fusion Pharmaceuticals Inc., Takeda, among others.
Liver Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key liver cancer companies, including Shanghai Henlius Biotech, Boehringer Ingelheim, Bristol-Myers Squibb, Jiangsu Hengrui Medicine, Glaxo SmithKline, ZAI Lab, Beijing Immunochina Medical Science and Technology, among others.
Advanced Liver Cancer Pipeline
Advanced Liver Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key advanced liver cancer companies, including Genoscience, Eureka Therapeutics, Celsion Corporation, H3 Biomedicine, TriSalus Life Sciences, Celldex Therapeutics, Takeda, Megapro Biomedical Company, Polaris Group, among others.
Advanced Liver Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key advanced liver cancer companies, including Genoscience, Eureka Therapeutics, Celsion Corporation, H3 Biomedicine, TriSalus Life Sciences, Celldex Therapeutics, Takeda, Megapro Biomedical Company, Polaris Group, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.


