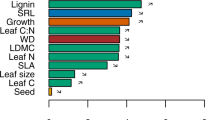
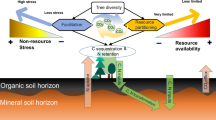
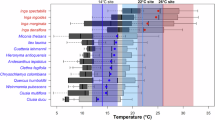
Abstract
Trees are an important carbon sink as they accumulate biomass through photosynthesis1. Identifying tree species that grow fast is therefore commonly considered to be essential for effective climate change mitigation through forest planting. Although species characteristics are key information for plantation design and forest management, field studies often fail to detect clear relationships between species functional traits and tree growth2. Here, by consolidating four independent datasets and classifying the acquisitive and conservative species based on their functional trait values, we show that acquisitive tree species, which are supposedly fast-growing species, generally grow slowly in field conditions. This discrepancy between the current paradigm and field observations is explained by the interactions with environmental conditions that influence growth. Acquisitive species require moist mild climates and fertile soils, conditions that are generally not met in the field. By contrast, conservative species, which are supposedly slow-growing species, show generally higher realized growth due to their ability to tolerate unfavourable environmental conditions. In general, conservative tree species grow more steadily than acquisitive tree species in non-tropical forests. We recommend planting acquisitive tree species in areas where they can realize their fast-growing potential. In other regions, where environmental stress is higher, conservative tree species have a larger potential to fix carbon in their biomass.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated in this study (EAN, TDN, SBD, TED) have been deposited in the https://entrepot.recherche.data.gouv.fr database (https://doi.org/10.57745/3OIGHB; Etalab Open License 2.0, compatible CC-BY 2.0). Data supporting Figs. 1–4 and Table 1 are provided in the Article and its Supplementary Information. There is no restriction on data availability. Source data are provided with this paper.
Code availability
Data were analysed using code developed by authors (R language, v.9.4 and v.4.2.3) and common statistical methods: random forest (randomForest R package, v.4.7-1.1), linear models (olsrr R package, v.0.5.3), mixed models (lme4 R package, v.1.1-32), Kruskal–Wallis test (R core). All analyses are fully described in the Methods. The main R procedures that were used have been deposited in the https://entrepot.recherche.data.gouv.fr database (https://doi.org/10.57745/3OIGHB). Complementary information is available from the authors on request.
References
Canadell, J. G. & Schulze, E. D. Global potential of biospheric carbon management for climate mitigation. Nat. Commun. 5, 5282 (2014).
Paine, C. E. T. et al. Globally, functional traits are weak predictors of juvenile tree growth, and we do not know why. J. Ecol. 103, 978–989 (2015).
IPCC Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Pörtner, H.-O. et al. (eds)) (2022).
Bonan, G. B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 (2008).
Tagesson, T. et al. Recent divergence in the contributions of tropical and boreal forests to the terrestrial carbon sink. Nat. Ecol. Evol. 4, 202–209 (2020).
Seidl, R., Schelhaas, M.-J., Rammer, W. & Verkerk, P. J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Change 4, 806–810 (2014).
Alkama, R. et al. Vegetation-based climate mitigation in a warmer and greener world. Nat. Commun. 13, 606 (2022).
Lambers, H. & Poorter, H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv. Ecol. Res. 23, 187–261 (1992).
Grime, J. et al. Integrated screening validates primary axes of specialisation in plants. Oikos 79, 259–281 (1997).
Herms, D. A. & Mattson, W. J. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335 (1992).
Laughlin, D. C. et al. Intraspecific trait variation can weaken interspecific trait correlations when assessing the whole-plant economic spectrum. Ecol. Evol. 7, 8936–8949 (2017).
Bongers, F. J. et al. Growth-trait relationships in subtropical forest are stronger at higher diversity. J. Ecol. 108, 256–266 (2020).
Wright, S. J. et al. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91, 3664–3674 (2010).
Herault, B. et al. Functional traits shape ontogenetic growth trajectories of rain forest tree species. J. Ecol. 99, 1431–1440 (2011).
Yang, J., Cao, M. & Swenson, N. G. Why functional traits do not predict tree demographic rates. Trends Ecol. Evol. 33, 326–336 (2018).
Gibert, A., Gray, E. F., Westoby, M., Wright, I. J. & Falster, D. S. On the link between functional traits and growth rate: meta-analysis shows effects change with plant size, as predicted. J. Ecol. 104, 1488–1503 (2016).
Weemstra, M., Zambrano, J., Allen, D. & Umaña, M. N. Tree growth increases through opposing above-ground and below-ground resource strategies. J. Ecol. 109, 3502–3512 (2021).
Augusto, L., Achat, D. L., Jonard, M., Vidal, D. & Ringeval, B. Soil parent material - a major driver of plant nutrient limitations in terrestrial ecosystems. Glob. Change Biol. 23, 3808–3824 (2017).
Nemani, R. R. et al. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300, 1560–1563 (2003).
Fisher, J. B., Badgley, G. & Blyth, E. Global nutrient limitation in terrestrial vegetation. Glob. Biogeochem. Cycles 26, GB3007 (2012).
Jonard, M. et al. Tree mineral nutrition is deteriorating in Europe. Glob. Change Biol. 21, 418–430 (2015).
Aerts, R. & Chapin, F. S. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv. Ecol. Res. 30, 1–67 (2000).
Chapin, F. S., Autumn, K. & Pugnaire, F. Evolution of suites of traits in response to environmental-stress. Am. Nat. 142, S78–S92 (1993).
Reich, P. B. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Song, C. et al. Differential tree demography mediated by water stress and functional traits in a moist tropical forest. Funct. Ecol. 37, 2927–2939 (2023).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Poorter, H., Lambers, H. & Evans, J. R. Trait correlation networks: a whole-plant perspective on the recently criticized leaf economic spectrum. N. Phytol. 201, 378–382 (2014).
Hunter, I. & Schuck, A. Increasing forest growth in Europe—possible causes and implications for sustainable forest management. Plant Biosyst. 136, 133–141 (2002).
Hoffmann, N., Heinrichs, S., Schall, P. & Vor, T. Climatic factors controlling stem growth of alien tree species at a mesic forest site: a multispecies approach. Eur. J. For. Res. 139, 915–934 (2020).
Van Sundert, K. et al. Towards comparable assessment of the soil nutrient status across scales—review and development of nutrient metrics. Glob. Change Biol. 26, 392–409 (2020).
Makoto, K., Kitagawa, R. & Blume-Werry, G. How do leaf functional traits and age influence the maximum rooting depth of trees? Eur. J. For. Res. 142, 1197–1206 (2023).
Koehler, K., Center, A. & Cavender-Bares, J. Evidence for a freezing tolerance-growth rate trade-off in the live oaks (Quercus series Virentes) across the tropical-temperate divide. N. Phytol. 193, 730–744 (2012).
Rueda, M., Godoy, O. & Hawkins, B. A. Trait syndromes among North American trees are evolutionarily conserved and show adaptive value over broad geographic scales. Ecography 41, 450–550 (2018).
Pierce, S. et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 31, 444–457 (2017).
Mirabel, A. et al. A whole-plant functional scheme predicting the early growth of tropical tree species: evidence from 15 tree species in Central Africa. Trees Struct. Funct. 33, 491–505 (2019).
Baez, S. & Homeier, J. Functional traits determine tree growth and ecosystem productivity of a tropical montane forest: insights from a long-term nutrient manipulation experiment. Glob. Change Biol. 24, 399–409 (2018).
Salgado-Luarte, C. & Gianoli, E. Shade tolerance and herbivory are associated with RGR of tree species via different functional traits. Plant Biol. 19, 413–419 (2017).
Bauman, D. et al. Tropical tree growth sensitivity to climate is driven by species intrinsic growth rate and leaf traits. Glob. Change Biol. 28, 1414–1432 (2022).
Serra-Maluquer, X. et al. Wood density and hydraulic traits influence species’ growth response to drought across biomes. Glob. Change Biol. 28, 3871–3882 (2022).
Salguero-Gomez, R. et al. Fast-slow continuum and reproductive strategies structure plant life-history variation worldwide. Proc. Natl Acad. Sci. USA 113, 230–235 (2016).
Francis, E. J. et al. Quantifying the role of wood density in explaining interspecific variation in growth of tropical trees. Glob. Ecol. Biogeogr. 26, 1078–1087 (2017).
Rodríguez-Alarcón, S., González-M, R., Carmona, C. P. & Tordoni, E. Trait-growth relationships in Colombian tropical dry forests: incorporating intraspecific variation and trait interactions. J. Veg. Sci. 35, e13233 (2024).
Huston, M. A. Precipitation, soils, NPP, and biodiversity: resurrection of Albrecht’s curve. Ecol. Monogr. 82, 277–296 (2012).
Townsend, A. R., Cleveland, C. C., Asner, G. P. & Bustamante, M. M. C. Controls over foliar N:P ratios in tropical rain forests. Ecology 88, 107–118 (2007).
Qin, Y. et al. Interactions between leaf traits and environmental factors help explain the growth of evergreen and deciduous species in a subtropical forest. For. Ecol. Manage. 560, 121854 (2024).
Prado-Junior, J. A. et al. Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 104, 817–827 (2016).
Felipe-Lucia, M. R. et al. Multiple forest attributes underpin the supply of multiple ecosystem services. Nat. Commun. 9, 4839 (2018).
Warner, E. et al. Young mixed planted forests store more carbon than monocultures—a meta-analysis. Front. For. Glob. Change 6, 1226514 (2023).
Baeten, L. et al. Identifying the tree species compositions that maximize ecosystem functioning in European forests. J. Appl. Ecol. 56, 733–744 (2019).
Yang, H. et al. Global increase in biomass carbon stock dominated by growth of northern young forests over past decade. Nat. Geosci. 16, 886–892 (2023).
Schwinning, S., Lortie, C. J., Esque, T. C. & DeFalco, L. A. What common-garden experiments tell us about climate responses in plants. J. Ecol. 110, 986–996 (2022).
Correia, A. H. et al. Early survival and growth plasticity of 33 species planted in 38 arboreta across the European Atlantic area. Forests 9, 630 (2018).
Manohan, B. et al. Use of functional traits to distinguish successional guilds of tree species for restoring forest ecosystems. Forests 14, 1075 (2023).
Paquette, A. et al. A million and more trees for science. Nat. Ecol. Evol. 2, 763–766 (2018).
Verheyen, K. et al. Contributions of a global network of tree diversity experiments to sustainable forest plantations. Ambio 45, 29–41 (2016).
Augusto, L. & Boča, A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 13, 1097 (2022).
Falster, D. S., Duursma, R. A. & FitzJohn, R. G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl Acad. Sci. USA 115, E6789–E6798 (2018).
Oktavia, D., Park, J. W. & Jin, G. Life stages and habitat types alter the relationships of tree growth with leaf traits and soils in an old-growth temperate forest. Flora 293, 152104 (2022).
Chen, G., Hobbie, S. E., Reich, P. B., Yang, Y. & Robinson, D. Allometry of fine roots in forest ecosystems. Ecol. Lett. 22, 322–331 (2019).
Enquist, B. J., Brown, J. H. & West, G. B. Allometric scaling of plant energetics and population density. Nature 395, 163–165 (1999).
Mokany, K., Raison, R. J. & Prokushkin, A. S. Critical analysis of root: shoot ratios in terrestrial biomes. Glob. Change Biol. 12, 84–96 (2006).
Ma, H. et al. The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nat. Ecol. Evol. 5, 1110–1122 (2021).
Niklas, K. J. & Spatz, H.-C. Growth and hydraulic (not mechanical) constraints govern the scaling of tree height and mass. Proc. Natl Acad. Sci. USA 101, 15661–15663 (2004).
Chiba, Y. Architectural analysis of relationship between biomass and basal area based on pipe model theory. Ecol. Modell. 108, 219–225 (1998).
Diaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016).
Kunstler, G. et al. Plant functional traits have globally consistent effects on competition. Nature 529, 204–209 (2016).
Wright, I. J. et al. Assessing the generality of global leaf trait relationships. N. Phytol. 166, 485–496 (2005).
Gomarasca, U. et al. Leaf-level coordination principles propagate to the ecosystem scale. Nat. Commun. 14, 3948 (2023).
Poorter, H., Remkes, C. & Lambers, H. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol. 94, 621–627 (1990).
Reich, P. B., Tjoelker, M., Walters, M., Vanderklein, D. & Buschena, C. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct. Ecol. 12, 327–338 (1998).
Doraisami, M. et al. A global database of woody tissue carbon concentrations. Sci. Data 9, 284 (2022).
Garnier, E., Shipley, B., Roumet, C. & Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 15, 688–695 (2001).
Perez-Harguindeguy, N. et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234 (2013).
Bruelheide, H. et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2, 1906–1917 (2018).
Caminha-Paiva, D., Negreiros, D., Barbosa, M. & Fernandes, G. W. Functional trait coordination in the ancient and nutrient-impoverished campo rupestre: soil properties drive stem, leaf and architectural traits. Biol. J. Linn. Soc. 133, 531–545 (2021).
Eviner, V. T. & Chapin, F. S. III Functional matrix: a conceptual framework for predicting multiple plant effects on ecosystem processes. Ann. Rev. Ecol. Evol. Syst. 34, 455–485 (2003).
Flores-Moreno, H. et al. Robustness of trait connections across environmental gradients and growth forms. Glob. Ecol. Biogeogr. 28, 1806–1826 (2019).
Osnas, J. L. D., Lichstein, J. W., Reich, P. B. & Pacala, S. W. Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 340, 741–744 (2013).
Reich, P. B. et al. The evolution of plant functional variation: traits, spectra, and strategies. Int. J. Plant Sci. 164, S143–S164 (2003).
de la Riva, E. G. et al. Root traits across environmental gradients in Mediterranean woody communities: are they aligned along the root economics spectrum? Plant Soil 424, 35–48 (2018).
Vet, R. et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 93, 3–100 (2014).
Hengl, T. et al. SoilGrids250m: global gridded soil information based on machine learning. PLoS ONE 12, e0169748 (2017).
Shangguan, W., Dai, Y., Duan, Q., Liu, B. & Yuan, H. A global soil data set for earth system modeling. J. Adv. Model. Earth Syst. 6, 249–263 (2014).
Lu, J. et al. Remarkable effects of microbial factors on soil phosphorus bioavailability: a country-scale study. Glob. Change Biol. 28, 4459–4471 (2022).
Toloşi, L. & Lengauer, T. Classification with correlated features: unreliability of feature ranking and solutions. Bioinformatics 27, 1986–1994 (2011).
Brienen, R. J. et al. Forest carbon sink neutralized by pervasive growth-lifespan trade-offs. Nat. Commun. 11, 4241 (2020).
Charru, M., Seynave, I., Hervé, J. C., Bertrand, R. & Bontemps, J. D. Recent growth changes in Western European forests are driven by climate warming and structured across tree species climatic habitats. Ann. For. Sci. 74, 33 (2017).
Harvey, J. E. et al. Tree growth influenced by warming winter climate and summer moisture availability in northern temperate forests. Glob. Change Biol. 26, 2505–2518 (2020).
Ols, C., Hervé, J.-C. & Bontemps, J.-D. Recent growth trends of conifers across Western Europe are controlled by thermal and water constraints and favored by forest heterogeneity. Sci. Total Environ. 742, 140453 (2020).
Lloyd, J. & Taylor, J. A. On the temperature-dependence of soil respiration. Funct. Ecol. 8, 315–323 (1994).
Adair, E. C. et al. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob. Change Biol. 14, 2636–2660 (2008).
Chen, S. et al. National estimation of soil organic carbon storage potential for arable soils: a data-driven approach coupled with carbon-landscape zones. Sci. Total Environ. 666, 355–367 (2019).
Kottek, M., Grieser, J., Beck, C., Rudolf, B. & Rubel, F. World map of the Koppen-Geiger climate classification updated. Meteorol. Z. 15, 259–263 (2006).
Chini, L. et al. LUH2-GCB2019: Land-Use Harmonization 2 Update For The Global Carbon Budget, 850-2019 (ORNL DAAC, 2021).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Hebbali, A. Olsrr: tools for building OLS regression models (2020); cran.r-project.org/package=olsrr.
Liaw, A. & Wiener, M. Classification and regression by randomForest. R News 2, 18–22 (2002).
Díaz-Uriarte, R. & Alvarez de Andrés, S. Gene selection and classification of microarray data using random forest. BMC Bioinform. 7, 3 (2006).
Shao, Z., Zhang, L. & Wang, L. Stacked sparse autoencoder modeling using the synergy of airborne LiDAR and satellite optical and SAR data to map forest above-ground biomass. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 10, 5569–5582 (2017).
Trefflich, I., Dietrich, S., Braune, A., Abraham, K. & Weikert, C. Short-and branched-chain fatty acids as fecal markers for microbiota activity in vegans and omnivores. Nutrients 13, 1808 (2021).
Babst, F. et al. Site- and species-specific responses of forest growth to climate across the European continent. Glob. Ecol. Biogeogr. 22, 706–717 (2013).
Poorter, L. et al. Biodiversity and climate determine the functioning of Neotropical forests. Glob. Ecol. Biogeogr. 26, 1423–1434 (2017).
Soong, J. L. et al. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci. Rep. 10, 2302 (2020).
van der Sande, M. T. et al. Soil fertility and species traits, but not diversity, drive productivity and biomass stocks in a Guyanese tropical rainforest. Funct. Ecol. 32, 461–474 (2018).
Noy-Meir, I., Walker, D. & Williams, W. Data transformations in ecological ordination: II. On the meaning of data standardization. J. Ecol. 63, 779–800 (1975).
Razali, N. M., Wah, Y. B. & others. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2, 21–33 (2011).
Hedges, L. V., Gurevitch, J. & Curtis, P. S. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999).
Malyjurek, Z., de Beer, D., Joubert, E. & Walczak, B. Working with log-ratios. Anal. Chim. Acta 1059, 16–27 (2019).
Voelkl, B., Würbel, H., Krzywinski, M. & Altman, N. The standardization fallacy. Nat. Methods 18, 5–7 (2021).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: global convergence in plant functioning. Proc. Natl Acad. Sci. USA 94, 13730–13734 (1997).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Ellsworth, D. S. et al. Convergence in phosphorus constraints to photosynthesis in forests around the world. Nat. Commun. 13, 5005 (2022).
Grime, J. P. & Hunt, R. Relative growth-rate: its range and adaptive significance in a local flora. J. Ecol. 63, 393–422 (1975).
Thomas, F. M. & Vesk, P. A. Are trait-growth models transferable? Predicting multi-species growth trajectories between ecosystems using plant functional traits. PLoS ONE 12, e0176959 (2017).
Bujang, M. A. & Baharum, N. Sample size guideline for correlation analysis. World J. Soc. Sci. Res. 3, 37–46 (2016).
Altman, N. & Krzywinski, M. Points of significance: association, correlation and causation. Nat. Methods 12, 899–900 (2015).
Altman, N. & Krzywinski, M. Analyzing outliers: influential or nuisance? Nat. Methods 13, 281–283 (2016).
West, P. A review of the growth behaviour of stands and trees in even-aged, monospecific forest. Ann. For. Sci. 81, 34 (2024).
Mayer, D. G. & Butler, D. G. Statistical validation. Ecol. Model. 68, 21–32 (1993).
Isaac, M. E. et al. Intraspecific trait variation and coordination: root and leaf economics spectra in coffee across environmental gradients. Front. Plant Sci. 8, 1196 (2017).
Kazakou, E. et al. Are trait-based species rankings consistent across data sets and spatial scales? J. Veg. Sci. 25, 235–247 (2014).
Treurnicht, M. et al. Functional traits explain the Hutchinsonian niches of plant species. Glob. Ecol. Biogeogr. 29, 534–545 (2020).
Ma, Z. et al. Evolutionary history resolves global organization of root functional traits. Nature 555, 94–97 (2018).
Pietsch, K. A. et al. Global relationship of wood and leaf litter decomposability: the role of functional traits within and across plant organs. Glob. Ecol. Biogeogr. 23, 1046–1057 (2014).
Joswig, J. S. et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 6, 36–50 (2022).
Fajardo, A. Insights into intraspecific wood density variation and its relationship to growth, height and elevation in a treeline species. Plant Biol. 20, 456–464 (2018).
Li, T. et al. Intraspecific functional trait variability across different spatial scales: a case study of two dominant trees in Korean pine broadleaved forest. Plant Ecol. 219, 875–886 (2018).
Pompa-García, M. et al. Tree-ring wood density reveals differentiated hydroclimatic interactions in species along a bioclimatic gradient. Dendrochronologia 85, 126208 (2024).
Ji, M., Jin, G. & Liu, Z. Effects of ontogenetic stage and leaf age on leaf functional traits and the relationships between traits in Pinus koraiensis. J. For. Res. 32, 2459–2471 (2021).
Kattge, J. et al. TRY plant trait database—enhanced coverage and open access. Glob. Change Biol. 26, 119–188 (2020).
Boehnke, M. & Bruelheide, H. How do evergreen and deciduous species respond to shade? Tolerance and plasticity of subtropical tree and shrub species of South-East China. Environ. Exp. Bot. 87, 179–190 (2013).
Cornelissen, J. A triangular relationship between leaf size and seed size among woody species: allometry, ontogeny, ecology and taxonomy. Oecologia 118, 248–255 (1999).
Unterholzner, L., Stolz, J., van der Maaten-Theunissen, M., Liepe, K. & van der Maaten, E. Site conditions rather than provenance drive tree growth, climate sensitivity and drought responses in European beech in Germany. For. Ecol. Manage. 572, 122308 (2024).
Ovenden, T. S., Jinks, R. L., Mason, W. L., Kerr, G. & Reynolds, C. A comparison of the early growth and survival of lesser-known tree species for climate change adaptation in Britain. For. Ecol. Manage. 572, 122340 (2024).
Albert, C. H., Grassein, F., Schurr, F. M., Vieilledent, G. & Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 13, 217–225 (2011).
Wooliver, R. C. et al. Phylogeny is a powerful tool for predicting plant biomass responses to nitrogen enrichment. Ecology 98, 2120–2132 (2017).
Zanne, A. E. et al. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014).
Lu, Y., Ran, J.-H., Guo, D.-M., Yang, Z.-Y. & Wang, X.-Q. Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS ONE 9, e107679 (2014).
Magallón, S., Gómez-Acevedo, S., Sánchez-Reyes, L. L. & Hernández-Hernández, T. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. N. Phytol. 207, 437–453 (2015).
Saladin, B. et al. Fossils matter: improved estimates of divergence times in Pinus reveal older diversification. BMC Evol. Biol. 17, 95 (2017).
Hipp, A. L. et al. Genomic landscape of the global oak phylogeny. N. Phytol. 226, 1198–1212 (2020).
Jiang, L. et al. Phylogeny and biogeography of Fagus (Fagaceae) based on 28 nuclear single/low-copy loci. J. Syst. Evol. 60, 759–772 (2022).
Liese, R., Alings, K. & Meier, I. C. Root branching is a leading root trait of the plant economics spectrum in temperate trees. Front. Plant Sci. 8, 315 (2017).
Cadotte, M. W., Davies, T. J. & Peres-Neto, P. R. Why phylogenies do not always predict ecological differences. Ecol. Monogr. 87, 535–551 (2017).
Augusto, L., Davies, T. J., Delzon, S. & de Schrijver, A. The enigma of the rise of angiosperms: can we untie the knot? Ecol. Lett. 17, 1326–1338 (2014).
Augusto, L. et al. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 90, 444–466 (2015).
Bond, W. The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 36, 227–249 (1989).
Brodribb, T. J. & Feild, T. S. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13, 175–183 (2010).
Brodribb, T. J., Pittermann, J. & Coomes, D. A. Elegance versus speed: examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 173, 673–694 (2012).
Martin, A. R., Doraisami, M. & Thomas, S. C. Global patterns in wood carbon concentration across the world’s trees and forests. Nat. Geosci. 11, 915–920 (2018).
Reich, P. B. et al. Evidence of a general 2/3-power law of scaling leaf nitrogen to phosphorus among major plant groups and biomes. Proc. R. Soc. B 277, 877–883 (2010).
Zheng, J. et al. A trait-based root acquisition-defence-decomposition framework in angiosperm tree species. Nat. Commun. 15, 5311 (2024).
Acknowledgements
The EAN common gardens were installed thanks to the financial support of the REINFFORCE project (INTERREG Atlantic Area; FCT grant number PD/BD/52405/2013 and 2008-1/005). Some of the EAN common gardens were then supported by European funding (LIFE IP CLIMAZ), national funding (Forestry Commission of the United Kingdom) or regional funding (Nouvelle Aquitaine region (France), Euskadi region (Spain), Sustainable Forest Management Research Institute (iuFOR; Spain)). The TDN common gardens were installed and monitored thanks to the financial support of different national agencies: the German Research Foundation (grants Ei 862/29-1, Ei 862/31-1, project no. 439223434) and the German Centre for Integrative Biodiversity Research (iDiv; grants DFG—FZT 118, 202548816); the BIOTREE experiment has been established by the Max-Planck-Institute for Biogeochemistry Jena, Germany, and its data collection in 2019 was supported by a grant to M.S.-L. by the German Research Foundation (DFG project number 439223434); the USA National Science Foundations (grants DEB-1234162, DEB−1831944, DEB-2106014, DEB-2044406); the USA NSERC-Discovery (grant RGPIN-2018-05201); and the Walloon Forest Service of Belgium. Other common gardens were supported by different funding, such as the AnaEE infrastructure (grant ANR-11-INBS-0001AnaEE-Services; France), the SoilSolution project (grant 41007-00210400; Finland), the Department of Geosciences and Natural Resource Management, University of Copenhagen and the Silva Nova project (grant NNF20OC0059948; Denmark). D.L.G. was supported by the EU Horizon project EXCELLENTIA (grant number 101087262) at Mendel University in Brno during the manuscript preparation phase. This study was funded by the CARTON project (grant ANR-19-CE32-0006), supported by the French Agence Nationale de la Recherche (ANR). We thank the landowners of the sites where the common gardens were installed; the colleagues who contributed to the installation of some of these common gardens: M. H. Almeida, M. Ferreira, S. Jorge, A. Nordin and P. Pastuszka; the numerous colleagues without whom it would have been impossible to install and maintain common gardens, and acquire data in the field, in particular, M. Belluau, S. Benham, O. Bouchez, V. Bouttier, T. Bouvet, M. Bustos, C. Chesseron, N. Cheval, R. Deblir, J. L. Denou, E. Diz, A. Don, C. Garbe, C. Gire, J. Haase, K. Hahner, B. Issenhuth, B. James, B. Bilde Jørgensen, F. Khalfallah, A. Kokko, B. Laffitte, J. Lakey, N. Laurent, D. Lesieur, P. Lhoir, F. Lyrou, D. Mackensen, T. Maxwell, M. El-Mazlouzi, M. Mörsdorf, S. Müller, C. Nock, M. Oram, A. Pazos, M. Pietrzak, F. Plume, O. Power, J. Pullen, A. Quintairos, J. Quosh, A. Reichard, L. Richardson, S. Stöckli, S. Thunot, S. R. Tziaferidis, J. Urgoiti, G. Xanthopoulos and all of the CNPF staff; the following colleagues, for their help during the measurement campaigns of functional traits: M. Aimaiti, A. Bosc, R. Burlett, N. Devert, J. C. Domec, T. Guzman, L. Jordan-Meille, F. Lagane, C. Lambrot, A. Loches, S. Milin and L. Wingate; A. Bourdin, P. Donoso, A. Fayolle, C. Lusk and A. Mirabel for providing data; G. Augusto-Sciama, M. Desailly, L. Fan, N. Fanin, F. Gosselin, R. Lemaire-Patin, C. Nguyen, A. Nys, E. Paturle, B. Ringeval and J. P. Wigneron for their help during data handling, data analyses and preparation of the manuscript; and our colleague, William ‘Bill’ Mason, who passed away before the publication of this study. Bill had a deep knowledge of forestry and substantially contributed to this work by questioning results, and discussing them in a very kind and constructive way.
Author information
Authors and Affiliations
Contributions
L.A. initiated the study and L.A. and M.C. designed it. L.A., M.C. and L.B. collected and curated data, with particular support from R.B., C.O. and N.G.-B. (EAN data) and A.B. (SBD data). Trait data were collected and consolidated by L.A., R.B., M.C. and M.R.B.; N.G.-B. and A.A.-G. provided soil data from the EAN sites. A.A.-G., N.G.-B., H.A., F.B., A.C., J.C.-B., A.H.C., A.D.S., J.J.D.-C., N.E., M.N.F., G.G., D.L.G., M.G.-C.-F., M.J.G., H.J., J.K., M.L., V.A.L., A.L., J.M.-G., W.L.M., C.M., S.M., R.A.M., B. Musch, B. Muys, E.P., A.P., J.D.P., W.C.P., Q.P., C.R., M.J.R.-L., R.R.-P., X.S.-I., M.S.-L., F.J.S.-P., A.S., G.S., E.B.T.-B., E.I.V., K.V. and L.V. provided data about tree growth from their respective common gardens. L.A., M.C. and R.B. made the first analysis and interpretation of data. L.A. wrote the first version of the manuscript, with the contribution of M.C. and R.B. Then L.A. revised the successive following versions of the manuscript with contributions from all of the authors, particularly M.C., R.B., M.R.B., M.J.G., B. Muys, C.M. and C.O.
Corresponding authors
Ethics declarations
Competing interests
R.B. is employed by a company that works with landowners to implement projects of reforestation or afforestation worldwide. F.J.S.-P. and M.J.R.-L. occasionally advise foresters or landowners. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Giuliano Locoselli, Stuart Wright and Pieter Zuidema for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Effect of specific leaf area (SLA) on seedling growth under favourable conditions.
Data were collected from 10 independent publications that reported seedling growth representing 263 species-experiments combinations and 212 distinct plant species (a), different forest biomes (b), plant types (c), and forested continents (d). A subset of values representing the tree species shared by the 10 publications about seedling growth and by the present study about trees is shown (e). Data are standardized to enable comparisons among sites (see Methods). Linear regressions were fitted by class of values. The SLA trait was chosen because it was studied in all the 10 publications (Supplementary Note 1) and because SLA is a key trait in the plant economics spectrum (ref. 24).
Extended Data Fig. 2
Context, rationale and hypothesis of the present study.
Extended Data Fig. 3 Effect of functional traits on tree growth.
Data were standardized to enable comparisons among sites (see Methods). Linear regressions were fitted (level of confidence of the error band = 0.95) by latitudinal class (limit values = 23° and 45°). P values of correlations are symbolized as follows: *** (P < 0.001), ** (P < 0.010), * (P < 0.050), (*) (P < 0.100), ns (P ≥ 0.100). For the scope of readability, a regression line and its data points are not presented together when several lines are shown in a given panel.
Extended Data Fig. 4 Effect of fine root traits on tree growth in the European Atlantic Network.
Fine roots are roots with a diameter ≤ 2 mm. Data are standardized to enable comparisons among sites (see Methods). For panels from a to h, the statistics of the regressions (level of confidence of the error band = 0.95) were: t = −1.3, +3.6, +9.6, −2.6, +0.7, −1.3, −5.2 and −3.6; df = 617 and n = 619 in all cases. Specific root length (SRL) results are presented in Extended Data Fig. 3. Original units: fine root content in carbon and nutrients (mg g−1), fine root dry matter content (g g−1), and fine root length density (cm−root cm−3−soil).
Extended Data Fig. 5 Relationships between photosynthetic capacity and growth at different latitudes.
Tree growth was quantified based on several metrics and values were standardized (see Methods). A linear regression was fitted (level of confidence of the error band = 0.95). Results are presented by latitude class: high-latitudes sites: |latitude| ≥ 45° (a); intermediate sites: 23° <|latitude| <45° (b); tropical sites: |latitude| ≤ 23° (c). For panels a, b, and c, the statistics of the regressions were respectively: t = −3.79, −2.83, and +0.88; df = 499, 434, and 135; n = 501, 436, and 137.
Extended Data Fig. 6 Examples of relationships between a functional trait and tree species growth.
Each panel presents the relationship between the Specific Leaf Area (SLA) value and the growth rate value of different tree species growing in a given common garden. Scatter plots present three sites of the European Atlantic Network (a, b, c) and three sites of the Tree Diversity Network (d, e, f). These sites are also identified in Fig. 2b. For panels from a-f, the statistics of the regressions (level of confidence of the error band = 0.95) were respectively: t = −2.13, −0.55, +1.85, −2.05, +1.33, and +3.40; df = 17, 18, 21, 10, 8, and 10; n = 19, 20, 23, 12, 10, and 12.
Extended Data Fig. 7 Influence of soil and climate on growth-trait relationships in the European Atlantic Network.
Linear regressions between growth rate and trait value were fitted for each site of the European Atlantic Network (see panels abc of Extended Data Fig. 6 for three examples of SLA-growth relationships). The correlation values (r) were then regressed to site productivity (i.e. the mean value of growth per site; Fig. 2). The fitted linear regression between site productivity and r values was finally used to draw the graph: for instance, the correlation between growth and trait value was systematically negative for wood density (WD; fitted linear regression in Fig. 2a), but switched from being negative at low productivity sites to positive at high productivity sites for SRL (Fig. 2d). Functional traits: Max height = tree species maximum height (m); Amax = maximum photosynthetic capacity (µmol g−1 s−1); SLA = specific leaf area (mm2 mg−1); SRL = specific root length (m g−1); WD = wood density (mg cm-3); Leaf N, Leaf P, and Root P = organ content in nitrogen or phosphorus (mg g−1).
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Supplementary Tables 1–4 and Supplementary Notes 1–8.
Supplementary Data
Source data for Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Augusto, L., Borelle, R., Boča, A. et al. Widespread slow growth of acquisitive tree species. Nature (2025). https://doi.org/10.1038/s41586-025-08692-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-08692-x